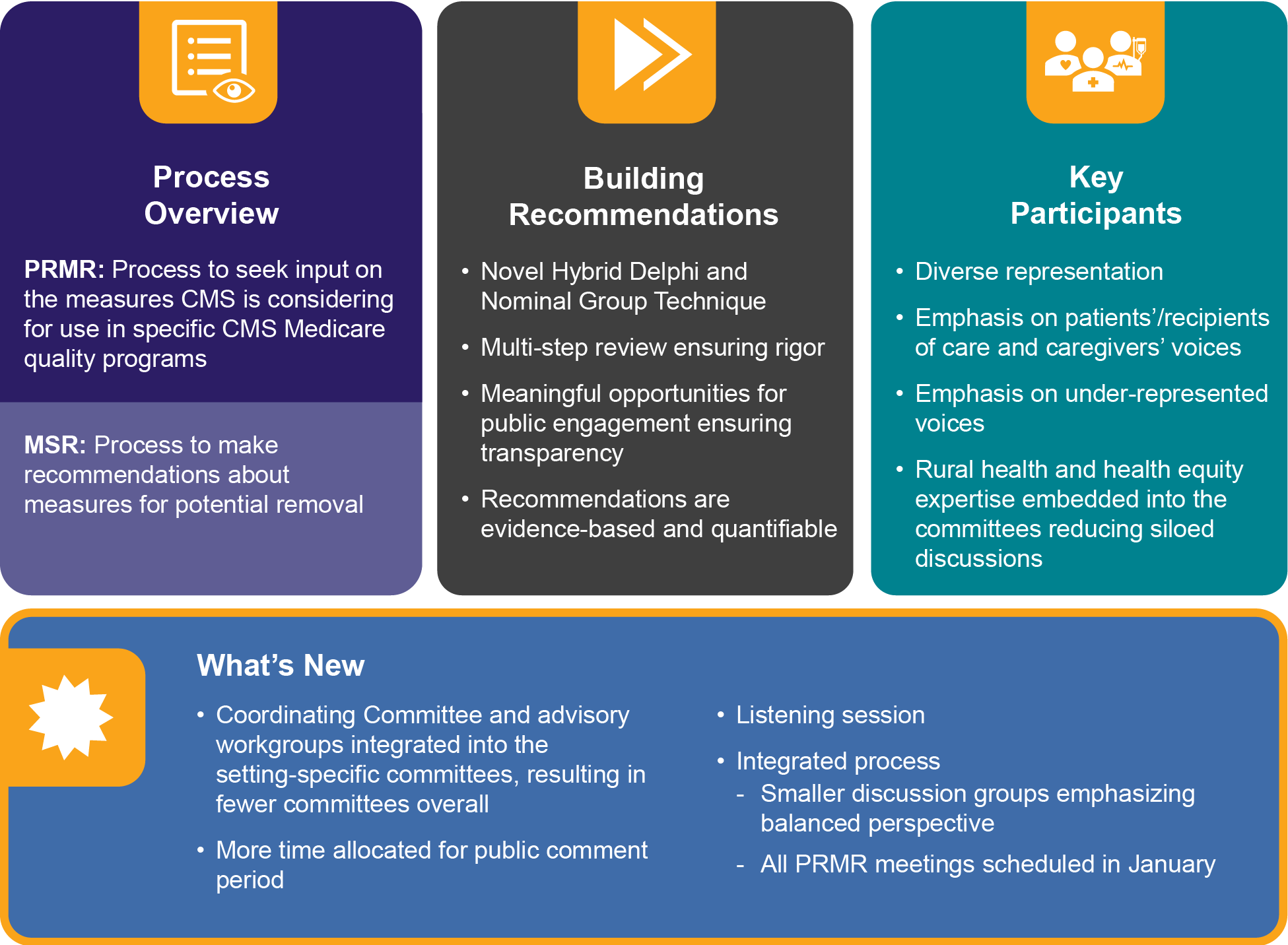
The MSR process is statutorily enabled by the Consolidated Appropriations Act, 2021 Public Law 116–260. PQM conducts the MSR annually to provide recommendations on the continued use of quality and efficiency measures in CMS programs.

Goal: To build recommendations around measure use to optimize the CMS measure portfolio in the quality reporting and value-based programs.
Each MSR cycle, a new area of focus will be selected with input from PQM membership and align with the Cascade of Meaningful Measures (Cascade). The Cascade is a tool to help prioritize existing health care quality measures, to align or reduce the number of measures, and identify gaps where new measures may need to be developed. Selection of a Cascade priority may be informed by conversations with key interested parties such as PRMR committee members, CMS, and other national policymakers and through environmental scans from conferences and other national health care priority activities.
In the context of a specific CMS program and population of beneficiaries, interested parties evaluate measures based on current information on the measure’s properties, performance trends in the CMS program, and whether the measure continues to support the program’s needs and priorities. Committee members consider three criteria in evaluation of measures:
- Meaningfulness
- Patient Journey
- Entity Data Stream Parsimony